L'interaction protéine-protéine : Hsp90 fongique
Les maladies fongiques entraînent annuellement environ 11 millions d'infections potentiellement mortelles. Les stratégies actuelles de traitement pour ces infections sont limitées par la résistance aux traitements antifongiques, leur toxicité et les interactions médicamenteuses.
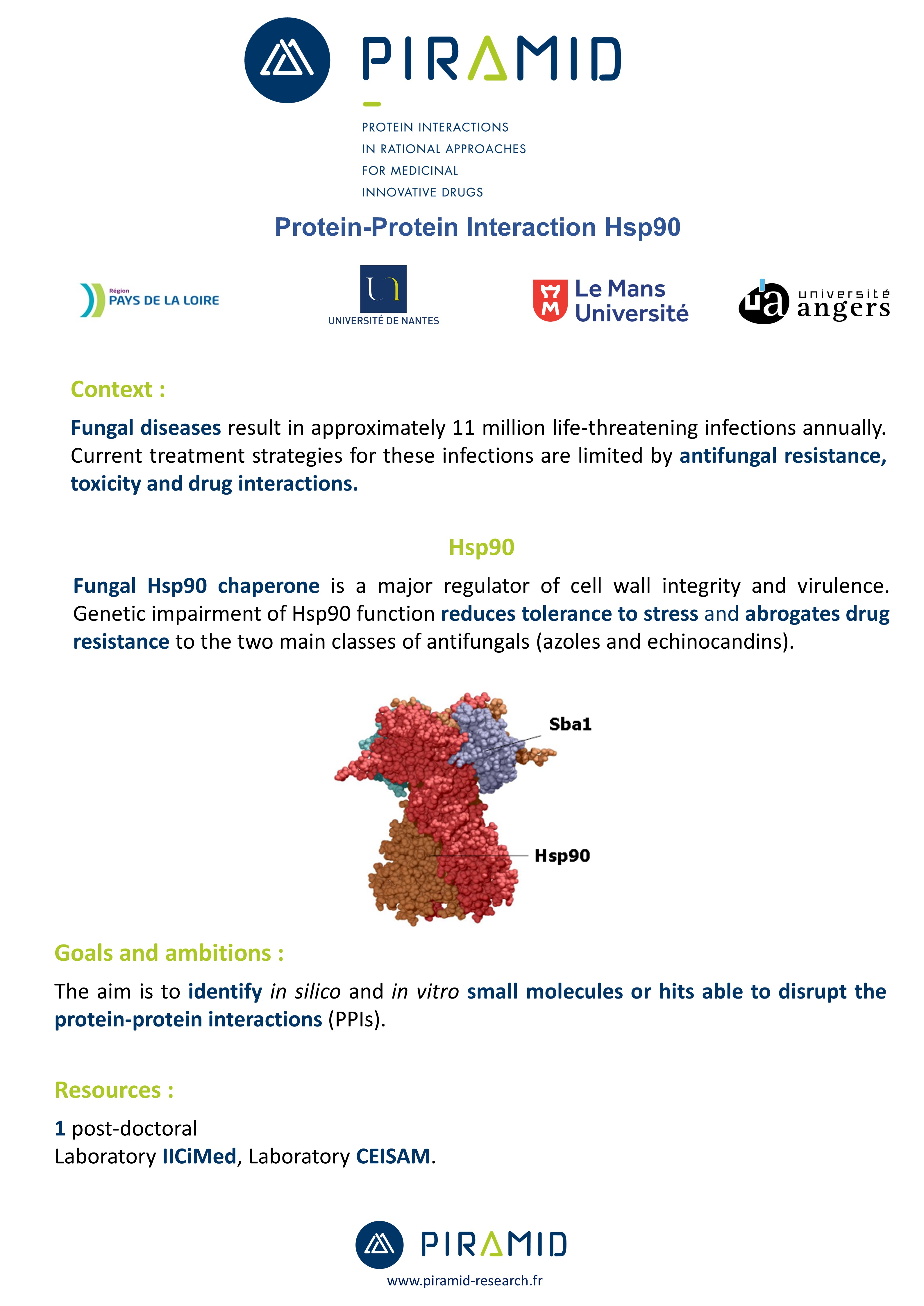
La protéine chaperon Hsp90 fongique est un régulateur majeur de l'intégrité de la paroi cellulaire et de la virulence. La déficience génétique des fonctions biologiques du chaperon moléculaire Hsp90 réduit la tolérance au stress et abroge la pharmacorésistance aux deux principales classes d'antifongiques (azolés et échinocandines). L’inhibition de Hsp90 par la geldanamycine restaure la sensibilité des souches de C. albicans résistantes aux composes azolés et aux échinocandines. Cependant, il a été montré que les inhibiteurs ATP-compétitifs d’Hsp90 ont un potentiel thérapeutique limité car ils présentent une toxicité in vivo en raison notamment de leur non sélectivité.
Ce projet propose une stratégie innovante et originale en mycologie médicale visant à inhiber sélectivement Hsp90 de levure en ciblant ses interactions avec les protéines associées (les co-chaperones). L’objectif est d’identifier in silico et in vitro des petites molécules ou hits capables de perturber les interactions protéine-protéine (IPP).
Cette approche sera réalisée grâce à un consortium interdisciplinaire réunissant des biologistes, des chimistes organiciens et des modélisateurs. Par conséquent, l'objectif fondamental et multidisciplinaire du projet sera l'identification de nouveaux candidats-médicaments ayant une activité antifongique sélective et susceptible de contourner la résistance aux médicaments antifongiques les plus largement utilisés.
Les membres/partenaires impliqués sont P. Le Pape (biologie), A. Laurent (modélisation moléculaire), P. Marchand et M.A. Bazin (chimie organique). Support de la plateforme IMPACT.
![]()
Protein-Protein Interactions:
Fungal Hsp90
Fungal diseases result in approximately 11 million life-threatening infections annually. Current treatment strategies for these infections are limited by antifungal resistance, toxicity and drug interactions.
Fungal Hsp90 chaperone is a major regulator of cell wall integrity and virulence. Genetic impairment of Hsp90 function reduces tolerance to stress and abrogates drug resistance to the two main classes of antifungals (azoles and echinocandins). Inhibition of Hsp90 by geldanamycin restores susceptibility of C. albicans strains resistant to azoles and echinocandins but such ATP competitive inhibitors appear to have limited therapeutic potential on their own as they exhibit in vivo toxicity because of their non-selectivity.
This project proposes an innovative and original strategy in medical mycology to selectively inhibit yeast Hsp90 by targeting its interactions with associated proteins. The aim is to identify in silico and in vitro small molecules or hits able to disrupt the protein-protein interactions (PPIs).
This approach will be carried out thanks to an interdisciplinary consortium, gathering biologists, organic and computational chemists. Therefore, the goal of the fundamental and multidisciplinary project will be the identification of new drug candidates with selective antifungal activity and being able to bypass resistance to the most widely used antifungal drugs.
The involved members/partners are P. Le Pape (Biology), A. Laurent (Molecular modeling), P. Marchand and M.A. Bazin (Organic chemistry). Support from the IMPACT plateforme.
English version Hsp90 PPI, Flyer and Poster, download here:
Téléchargez ici le flyer de la PPI Hsp90 en version française :